Cysview® (hexaminolevulinate HCl) is the only FDA-approved agent for use in Blue Light Cystoscopy (BLC®).
It is an optical imaging agent indicated for the detection of non-muscle invasive bladder cancer, including carcinoma in situ (CIS), in patients:
- suspected or known to have lesion(s) based on a prior cystoscopy or
- undergoing surveillance cystoscopy for carcinoma of the bladder.
Cysview makes tumor cells glow bright pink in blue light, but it is not a dye. It is a variant of a naturally occurring molecule in the body that results in increased production of another natural compound. Unhealthy cells do not process out the compound as quickly as healthy cells; the resulting accumulation creates a pink glow in blue light.
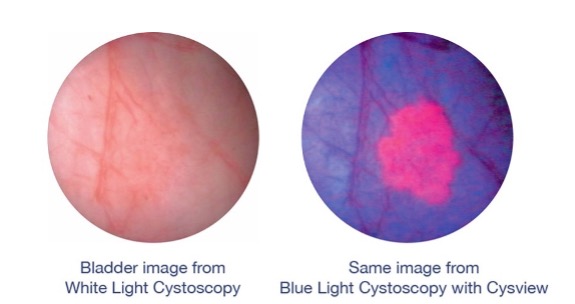
During a standard cystoscopy procedure, a urology healthcare professional examines the bladder using regular white light. During a Blue Light Cystoscopy, the HCP uses both white and blue light. However, the blue light is only effective if Cysview has been instilled in the patient’s bladder at least one hour before the procedure.
BLC with Cysview has been used with over 500,000 patients worldwide. It was approved in the U.S. in 2010 and was included in the American Urological Association (AUA) and Society of Urologic Oncology (SUO) guideline for the management of patients with non-muscle invasive bladder cancer in 2016 (amended 2020). Over 175 centers in the U.S. use BLC with Cysview.
Atlantic Urology is one of only 21 locations in the U.S. that currently offer flexible BLC with Cysview in the office. The others use it in the operating room with a rigid cystoscope.