We are proud to have his incredible dedication and expertise on our team!
As published in the New England Journal of Medicine (click here for original article)
Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide
Abstract
BACKGROUND
Darolutamide is a structurally distinct androgen-receptor inhibitor that is approved for the treatment of nonmetastatic, castration-resistant prostate cancer. In the planned primary analysis of a phase 3 trial, the median metastasis-free survival was significantly longer with darolutamide (40.4 months) than with placebo (18.4 months). The data for the analysis of overall survival were immature at the time of the primary analysis.
METHODS
In this double-blind, placebo-controlled trial, we randomly assigned 1509 men, in a 2:1 ratio, to receive darolutamide (955 patients) or placebo (554 patients) while they continued to receive androgen-deprivation therapy. After the results of the primary end-point analysis were found to be positive, unblinding of the treatment assignments occurred, and patients in the placebo group were permitted to cross over to receive open-label darolutamide treatment. At the time of this prespecified final analysis, which had been planned to be performed after approximately 240 deaths had occurred, overall survival and all other secondary end points were evaluated.
RESULTS
The median follow-up time was 29.0 months. At the time of unblinding of the data, all 170 patients who were still receiving placebo crossed over to receive darolutamide; 137 patients who had discontinued placebo before unblinding had occurred received at least one other life-prolonging therapy. Overall survival at 3 years was 83% (95% confidence interval [CI], 80 to 86) in the darolutamide group and 77% (95% CI, 72 to 81) in the placebo group. The risk of death was significantly lower, by 31%, in the darolutamide group than in the placebo group (hazard ratio for death, 0.69; 95% CI, 0.53 to 0.88; P=0.003). Darolutamide was also associated with a significant benefit with respect to all other secondary end points, including the time to first symptomatic skeletal event and the time to first use of cytotoxic chemotherapy. The incidence of adverse events after the start of treatment was similar in the two groups; no new safety signals were observed.
CONCLUSIONS
Among men with nonmetastatic, castration-resistant prostate cancer, the percentage of patients who were alive at 3 years was significantly higher among those who received darolutamide than among those who received placebo. The incidence of adverse events was similar in the two groups. (Funded by Bayer HealthCare and Orion Pharma; ARAMIS ClinicalTrials.gov number, NCT02200614. opens in new tab.)
Nonmetastatic, castration-resistant prostate cancer is defined by rising levels of serum prostate-specific antigen (PSA) and an absence of detectable metastases on conventional imaging in patients receiving androgen-deprivation therapy.1,2 Patients with nonmetastatic, castration-resistant prostate cancer are at risk for progression to metastatic disease,3 which is often accompanied by the onset of cancer-related symptoms in this previously asymptomatic population.4 Prolonging survival and delaying the onset of cancer-related symptoms while minimizing treatment-related adverse events are key therapeutic goals in patients with nonmetastatic, castration-resistant prostate cancer.5
Darolutamide, a structurally distinct androgen-receptor inhibitor, is approved for the treatment of nonmetastatic, castration-resistant prostate cancer6 on the basis of the current trial — the phase 3 Androgen Receptor Antagonizing Agent for Metastasis-free Survival (ARAMIS) trial — in which the addition of darolutamide to ongoing androgen-deprivation therapy significantly prolonged median metastasis-free survival by 22 months.7 As expected, the overall survival data were immature at the time of the primary analysis for metastasis-free survival, with the occurrence of only 136 deaths; the final analysis was planned to be performed after the occurrence of approximately 240 deaths. Nonetheless, the interim analysis for overall survival favored darolutamide over placebo (hazard ratio for death, 0.71; 95% confidence interval [CI], 0.50 to 0.99; P=0.045), although the prespecified significance level of 0.0002 was not reached.7 Darolutamide was not associated with a higher incidence of adverse events that are known to be associated with other androgen-receptor inhibitors — including falls, seizures, cognitive disorders, mental impairment disorders, and hypertension — than placebo.8-10 Quality of life was maintained for the duration of treatment.7 We report here the results from the prespecified final analysis of overall survival, all other secondary end points, and long-term safety in the ARAMIS trial.
Methods
TRIAL DESIGN AND CONDUCT
The trial was sponsored by Bayer HealthCare and Orion Pharma. Both sponsors, together with the first and last authors, developed the trial design. The institutional review board at each participating institution approved the trial, which was conducted in compliance with the principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice. All the patients provided written informed consent. Unblinded safety data were reviewed by an independent data and safety monitoring board throughout the trial.
The data were collected by the investigators, analyzed by statisticians who were employed by the sponsors, and interpreted by the authors, including employees of the sponsors. Bayer HealthCare provided funding for medical writing and editing assistance. The authors reviewed and approved the manuscript that was submitted for publication. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and statistical analysis plan, available with the full text of this article at NEJM.org.
PATIENTS
Full details of the trial inclusion and exclusion criteria have been reported previously.7 In brief, men who had nonmetastatic, castration-resistant prostate cancer and a baseline PSA level of at least 2 ng per milliliter, a PSA doubling time of 10 months or less, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 (scores range from 0 to 5, with higher scores indicating greater disability) were eligible for participation. Enrollment of patients who had a previous seizure disorder or a condition that conferred a predisposition to seizure was permitted.
TRIAL DESIGN AND TREATMENT ASSIGNMENTS
Patients were randomly assigned, in a 2:1 ratio in a double-blind manner, to receive darolutamide at an oral dose of 600 mg twice daily with food or matched placebo while they continued to receive androgen-deprivation therapy. Randomization was stratified according to PSA doubling time (≤6 months vs. >6 months) and the use of osteoclast-targeted therapy at randomization (yes vs. no). Patients continued to take darolutamide or placebo until protocol-defined progression, discontinuation of the assigned treatment because of adverse events, start of another anticancer therapy, or withdrawal of consent. Unblinding of the treatment assignments occurred after the results from the primary analysis for metastasis-free survival were found to be positive, at which time patients in the placebo group were allowed to cross over to receive open-label darolutamide treatment or to receive other subsequent treatment at the discretion of the investigator (Figs. S1 and S2 in the Supplementary Appendix, available at NEJM.org).
ASSESSMENTS
Information on demographic characteristics, relevant medical history, and pertinent clinical conditions was obtained at the screening visit, as described previously.7 Data were collected at 16-week intervals during the double-blind treatment period, at the start of the open-label treatment period, and every 16 weeks thereafter until the end of the trial. If metastatic progression was observed by the investigator, the trial treatment was discontinued, and the patient was followed every 16 weeks until death or the end of the trial. At the time of progression, treatment outside the trial protocol could be initiated at the discretion of the physician. Review of imaging results was performed both locally and by blinded independent central review during the double-blind period but was performed only locally during the open-label period. Assessment of laboratory values, including the PSA level, was performed centrally during both treatment periods. Data on adverse events that occurred after the start of treatment, including the type and severity (graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0311) of the events, as well as whether they were assessed by the investigator as being related to the trial treatment, were recorded at each visit.
END POINTS
The results of the analysis of the primary end point, metastasis-free survival, have been reported previously.7 Secondary end points evaluated at this final analysis included overall survival, the time to pain progression, the time to first use of cytotoxic chemotherapy, and the time to first symptomatic skeletal event. Pain progression was defined as either an increase of 2 or more points from baseline in the score assessed with the Brief Pain Inventory Short-Form questionnaire (a 10-point scale on which higher numbers reflect greater pain; minimum clinically important difference, 2 points) or initiation of opioid treatment for cancer pain, whichever occurred first. A symptomatic skeletal event was defined as external-beam radiation therapy to relieve skeletal symptoms, a new symptomatic pathologic bone fracture, the occurrence of spinal cord compression, or tumor-related orthopedic surgical intervention. Exploratory end points evaluated at this final analysis include the time to first prostate cancer–related invasive procedure and the time to initiation of subsequent antineoplastic therapy.
STATISTICAL ANALYSIS
The test for statistical significance of the final analysis was planned to be performed after approximately 240 deaths had occurred. Secondary end points were evaluated in a hierarchical order, with a two-sided significance level of 0.05 split between the primary analysis (0.0002) and the final analysis (0.0498) by a rho-family spending function with a parameter rho of 10; overall survival, the time to pain progression, the time to first use of cytotoxic chemotherapy, and the time to first symptomatic skeletal event were tested sequentially.
A stratified log-rank test with the same stratification factors as those used for randomization was used to compare the darolutamide and placebo groups. Kaplan–Meier curves, including median survival times and corresponding 95% confidence intervals, were constructed for all secondary and exploratory end points; hazard ratios were calculated in time-to-event analyses with the use of Cox proportional-hazards models. Prespecified subgroup analyses of overall survival were performed to determine the effect of demographic or baseline characteristics.
The statistical analysis and the generation of patient data listings were performed with the use of SAS software, version 9.2 (SAS Institute). Incomplete data on event occurrence dates were imputed as the earliest possible date.
Efficacy was evaluated in the intention-to-treat population, which comprised all patients who underwent randomization. Safety was evaluated in the safety population, which comprised all patients who underwent randomization and received at least one dose of darolutamide or placebo. Adverse events were assessed separately for the double-blind period and the open-label period. If the start date of an adverse event was missing, the adverse event was considered to have occurred during the double-blind period.
Results
PATIENTS
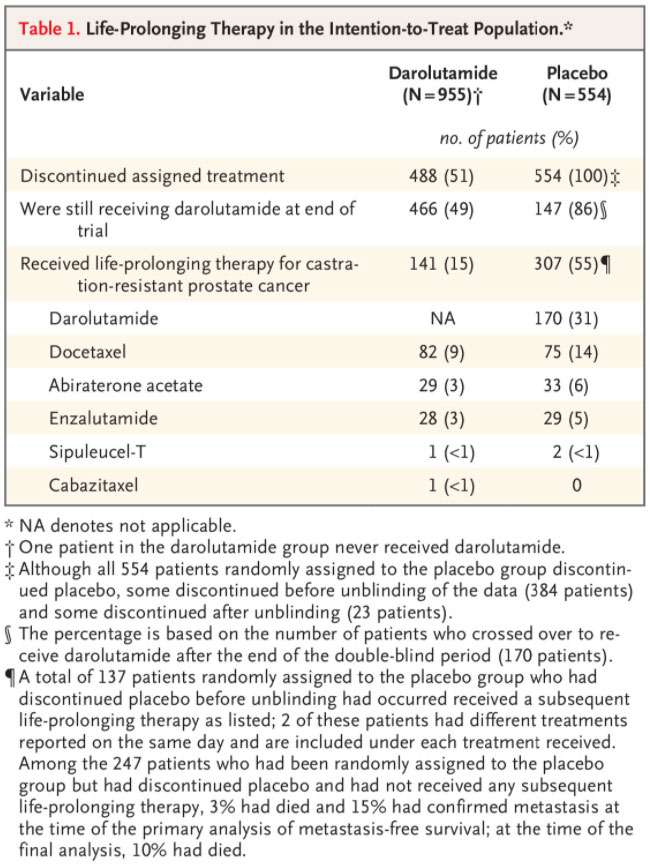
Patients were enrolled from September 2014 through March 2018. The intention-to-treat population included 1509 patients (955 in the darolutamide group and 554 in the placebo group) (Fig. S2). Demographic and clinical characteristics of the two groups were well balanced at baseline, as reported previously.7 In addition, the primary analysis of metastasis-free survival showed that, among the patients who had metastatic progression, the distribution of sites of metastases was similar in the darolutamide and placebo groups (Table S1).
The data cutoff for the primary analysis was September 3, 2018, and the unblinding of the treatment assignments occurred on November 30, 2018 (Fig. S1). At the time of unblinding, all 170 patients who were still receiving placebo crossed over to receive open-label darolutamide (crossover group) (Fig. S2). The data cutoff for the final analysis of overall survival was November 15, 2019; the median follow-up time was 29.0 months for the overall trial population, 11.2 additional months after the primary analysis of metastasis-free survival. At the time of data cutoff, 49% of the patients who had been originally assigned to receive darolutamide were still receiving darolutamide, and 86% of the patients in the crossover group were still receiving darolutamide (Table 1). The median duration of exposure in the darolutamide group was 25.8 months during the combined double-blind and open-label periods. In the placebo group, the median duration of exposure during the double-blind period was 11.6 months. Patients who crossed over to receive open-label darolutamide had a median duration of exposure to darolutamide of 11.0 months.
SUBSEQUENT THERAPY
In the placebo group, 307 of the 554 patients (55%) received subsequent treatment with darolutamide or other life-prolonging therapy (Table 1). Among the 384 patients randomly assigned to the placebo group who had discontinued placebo before unblinding of the data had occurred, 137 received a subsequent life-prolonging therapy other than darolutamide. The most commonly used subsequent life-prolonging therapies were docetaxel, abiraterone acetate, and enzalutamide. In the darolutamide group, 141 of the 955 patients (15%) received subsequent life-prolonging therapy other than darolutamide.
OVERALL SURVIVAL

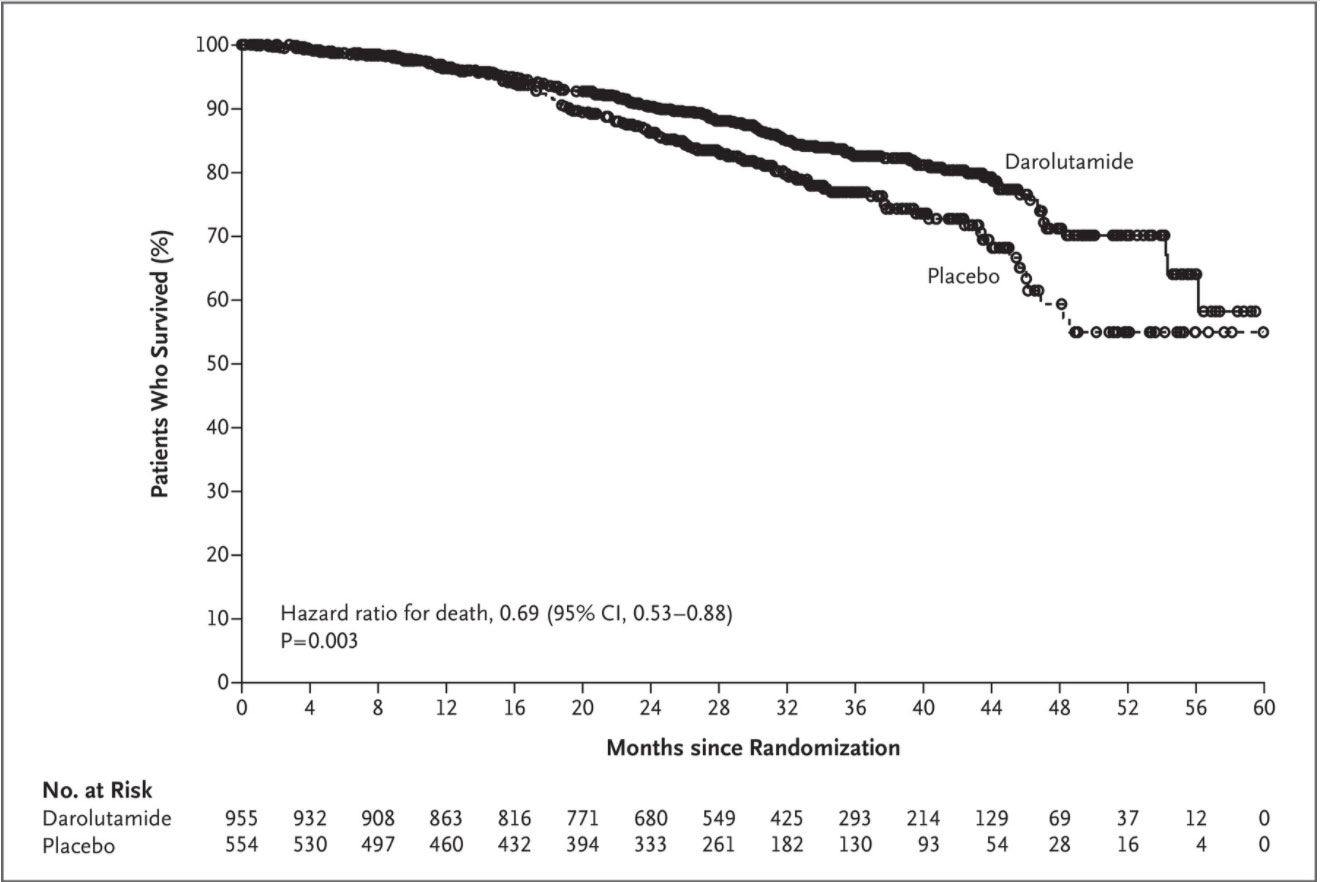
The final analysis of overall survival was performed after 254 deaths (148 [15%] in the darolutamide group and 106 [19%] in the placebo group) had occurred. The percentage of patients who were alive at 3 years was 83% (95% CI, 80 to 86) in the darolutamide group and 77% (95% CI, 72 to 81) in the placebo group. The risk of death was significantly lower, by 31%, in the darolutamide group than in the placebo group (hazard ratio for death, 0.69; 95% CI, 0.53 to 0.88; P=0.003) (Table 2 and Figure 1). In both treatment groups, the number of prostate cancer–related deaths was higher than the number of deaths from any other cause (80 of 149 deaths in the darolutamide group and 56 of 106 deaths in the placebo group) (Table S2), and the majority of those deaths occurred after discontinuation of treatment. However, the trial was not powered to assess the treatment effect of darolutamide on deaths due to prostate cancer.
The treatment effect on overall survival consistently favored darolutamide over placebo in prespecified subgroups, including those defined according to baseline PSA doubling time of 6 months or less or more than 6 months, geographic region, presence or absence of lymph-node involvement at baseline, and baseline ECOG performance status score of 0 or 1 (although the confidence intervals in some subgroups with a smaller sample size crossed 1.00) (Fig. S3).
OTHER SECONDARY AND EXPLORATORY END POINTS
Because statistical significance for overall survival was achieved, the time to pain progression, the time to first use of cytotoxic chemotherapy, and the time to first symptomatic skeletal event were evaluated hierarchically in this sequence. The time to pain progression was evaluated with the use of data from the primary analysis cutoff date of September 3, 2018, since no additional data were collected for this end point beyond that time; the analysis showed a significantly longer time to pain progression in the darolutamide group than in the placebo group (median, 40.3 months vs. 25.4 months; hazard ratio, 0.65; 95% CI, 0.53 to 0.79; P<0.001).
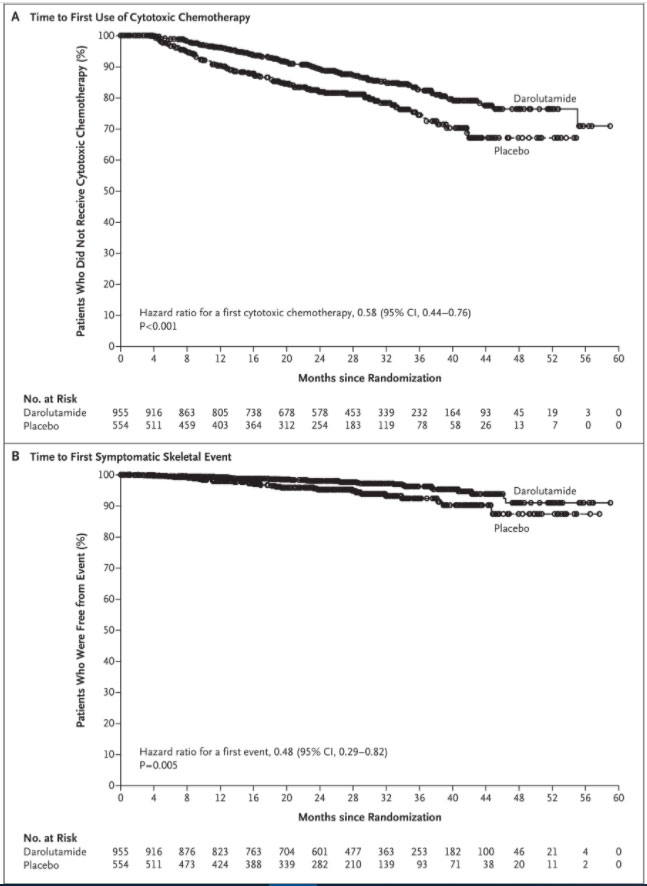
At 3 years, the percentage of patients who had not yet received their first cytotoxic chemotherapy was 83% in the darolutamide group and 75% in the placebo group. Darolutamide was associated with a significantly longer time to first use of cytotoxic chemotherapy than placebo (hazard ratio, 0.58; 95% CI, 0.44 to 0.76; P<0.001) (Table 2 and Figure 2). At 3 years, the percentage of patients who had not had a first symptomatic skeletal event was 96% in the darolutamide group and 92% in the placebo group. Darolutamide was associated with a significantly longer time to first symptomatic skeletal event (hazard ratio, 0.48; 95% CI, 0.29 to 0.82; P=0.005) than placebo (Table 2 and Figure 2).
The results of the exploratory end points evaluated at this analysis also favored darolutamide over placebo (Table 2). Treatment with darolutamide resulted in a longer time to disease progression and a longer time to additional treatment (i.e., prostate cancer–related invasive procedures and subsequent antineoplastic therapy).
SAFETY
Overall, adverse events were reported in 85.7% of the patients who received darolutamide and in 79.2% of the patients who received placebo during the double-blind period, as well as in 70.0% of the patients in the crossover group during the open-label period (Table S3). The percentages of patients who discontinued the assigned treatment because of adverse events were unchanged from the primary analysis (8.9% in the darolutamide group and 8.7% in the placebo group). The incidences of serious adverse events and grade 5 adverse events in the darolutamide group and the placebo group during the double-blind period were consistent with those from the primary analysis. With longer exposure to darolutamide, the incidence of all types of adverse events increased slightly, as expected. The types of adverse events reported in the crossover group were in line with those observed with darolutamide treatment.

The results of the final analysis of the safety profile of darolutamide are consistent with those of the primary analysis reported previously. Fatigue was reported in 13.2% of the patients in the darolutamide group and was the only adverse event reported in more than 10% of the patients in that group during the double-blind period (Table S4). The incidence of all other adverse events that occurred in more than 5% of the patients in either group was generally similar in the two groups. The results of the analysis of key adverse events that are known to be associated with androgen-deprivation therapy continued to show small or no differences in incidence between the darolutamide group and the placebo group (Table 3). The incidence of cardiac events is represented by the grouped terms of cardiac arrhythmia, coronary-artery disorder, and heart failure. Although the incidence of cardiac arrhythmia was higher with darolutamide than with placebo, both a history of cardiac arrhythmia and electrocardiographic abnormalities were present to a greater extent in the darolutamide group at baseline, as observed at the time of the primary analysis (unpublished data). After adjustment for treatment exposure, the incidences of most of the adverse events of interest, including falls, seizures, hypertension, mental-impairment disorders, and depressed-mood disorders, showed little or no difference between the darolutamide group and the placebo group (Table 3).
Discussion
At this prespecified final analysis, the percentage of patients who were alive at 3 years was 83% with darolutamide and 77% with placebo. Among men with nonmetastatic, castration-resistant prostate cancer and a PSA doubling time of 10 months or less, darolutamide was associated with a significant overall survival benefit, with a 31% lower risk of death than placebo. The survival benefit with darolutamide was evident from the Kaplan–Meier survival curves, which showed a separation of the two treatment groups at 18 months, favoring darolutamide, and this separation was maintained over time. This overall survival benefit was observed despite the fact that more than half the patients in the placebo group (307 of the 554 patients) received subsequent treatment with darolutamide or other life-prolonging therapy. This treatment effect was also consistent across patient subgroups. In addition, darolutamide was associated with a significant benefit with respect to all other secondary end points. As compared with placebo, treatment with darolutamide resulted in a significantly longer time to first use of cytotoxic chemotherapy, a significantly longer time to first symptomatic skeletal event, and a significantly longer time to pain progression.
After a median follow-up of 29.0 months in the overall trial population, darolutamide continued to have a favorable safety profile, similar to that described previously.7 The percentage of patients who discontinued the assigned treatment because of adverse events during the complete double-blind period was unchanged from that reported at the primary analysis, indicating that patients with nonmetastatic, castration-resistant prostate cancer are able to receive this androgen-receptor inhibitor for prolonged periods. The incidence of adverse events commonly associated with androgen-receptor inhibitors, including falls, seizures, mental-impairment disorders, and hypertension, was similar in the two groups. The incidence of fractures was slightly higher in the darolutamide group than in the placebo group; however, after adjustment for the duration of exposure, the between-group difference decreased. This updated analysis of the ARAMIS trial confirms the low potential for central nervous system–related effects expected with darolutamide, which has been postulated to be due to the very low penetration of the blood–brain barrier that has been reported in preclinical and clinical studies of darolutamide.12,13 Consideration of the safety profiles of treatments for nonmetastatic, castration-resistant prostate cancer is important for assessing the risk–benefit balance for patients. In clinical trials, other androgen-receptor inhibitors have shown higher incidences of central nervous system–related adverse events and hypertension than placebo.9,10,14 The patient population in the current trial generally had a lower incidence of adverse events than did the patients in the PROSPER and SPARTAN (Selective Prostate Androgen Receptor Targeting with ARN-509) trials.7,9,10 In all three trials, there were differences in the duration of treatment and follow-up that could have directly affected the incidence of adverse events reported in the placebo group of each trial. The fact that the incidence of death in the placebo group in the current trial was higher than the incidence of any individual adverse event is not surprising, given the median age of the trial population (74 years) and the percentage of patients who had coexisting conditions (98% of patients).
A major strength of this trial is the large size, which enabled a robust statistical analysis, particularly for the reported overall survival analysis with extended follow-up. A limitation of the trial is the small size of some subgroups, and hence, the low numbers of events in these subgroups and the low numbers of patients of particular races or ethnic groups (e.g., only 52 patients of African descent); therefore, no conclusions can be drawn about efficacy in these specific groups of patients.
Metastasis-free survival and overall survival were significantly longer with darolutamide than with placebo among men with nonmetastatic, castration-resistant prostate cancer and a PSA doubling time of 10 months or less. An overall survival benefit was observed even though more than half the patients in the placebo group received subsequent treatment with darolutamide or another life-prolonging therapy. The time to first use of cytotoxic chemotherapy, the time to first symptomatic skeletal event, and the time to pain progression were significantly longer with darolutamide than with placebo. The incidence of adverse events was similar in the darolutamide group and the placebo group.